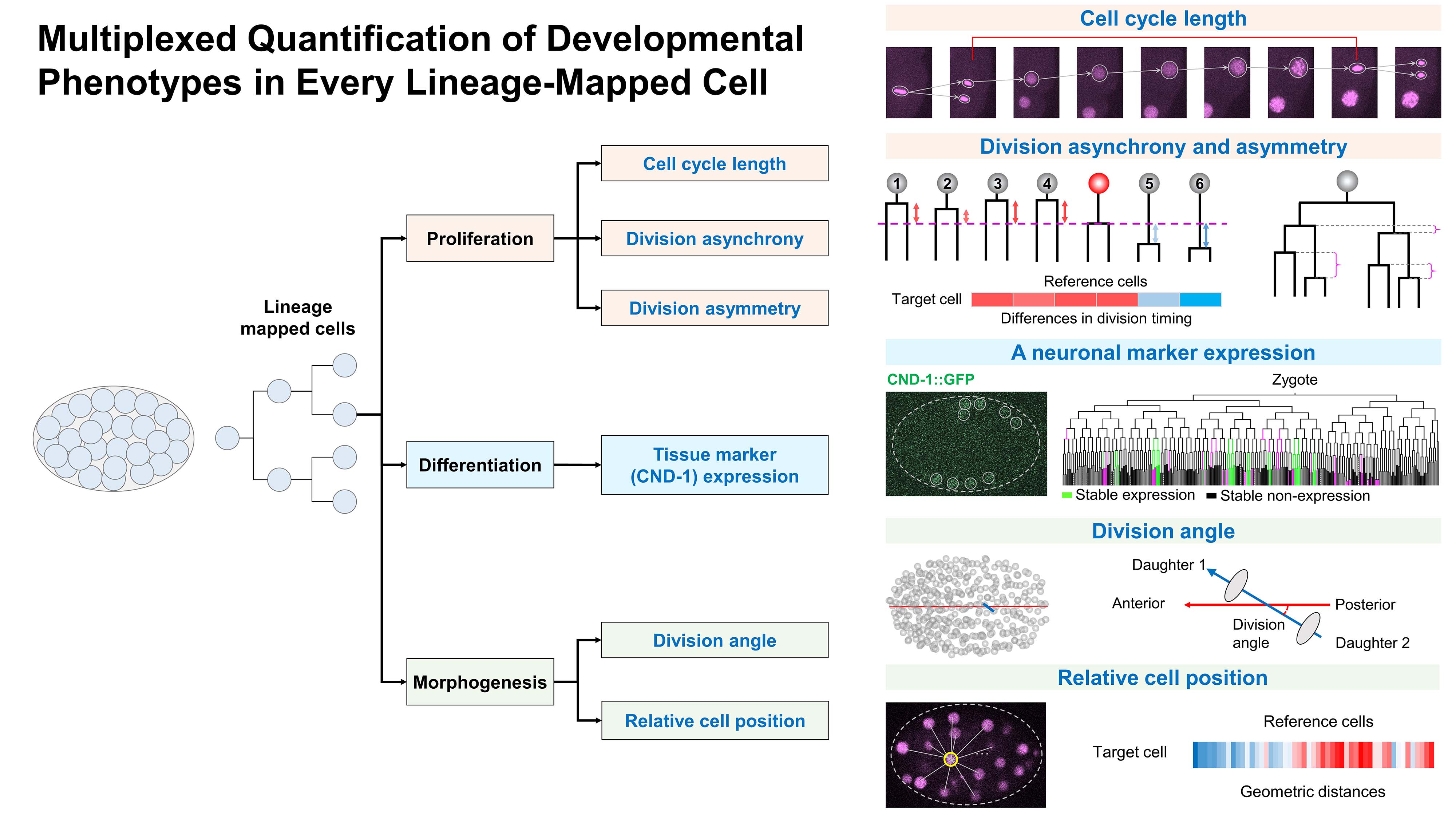
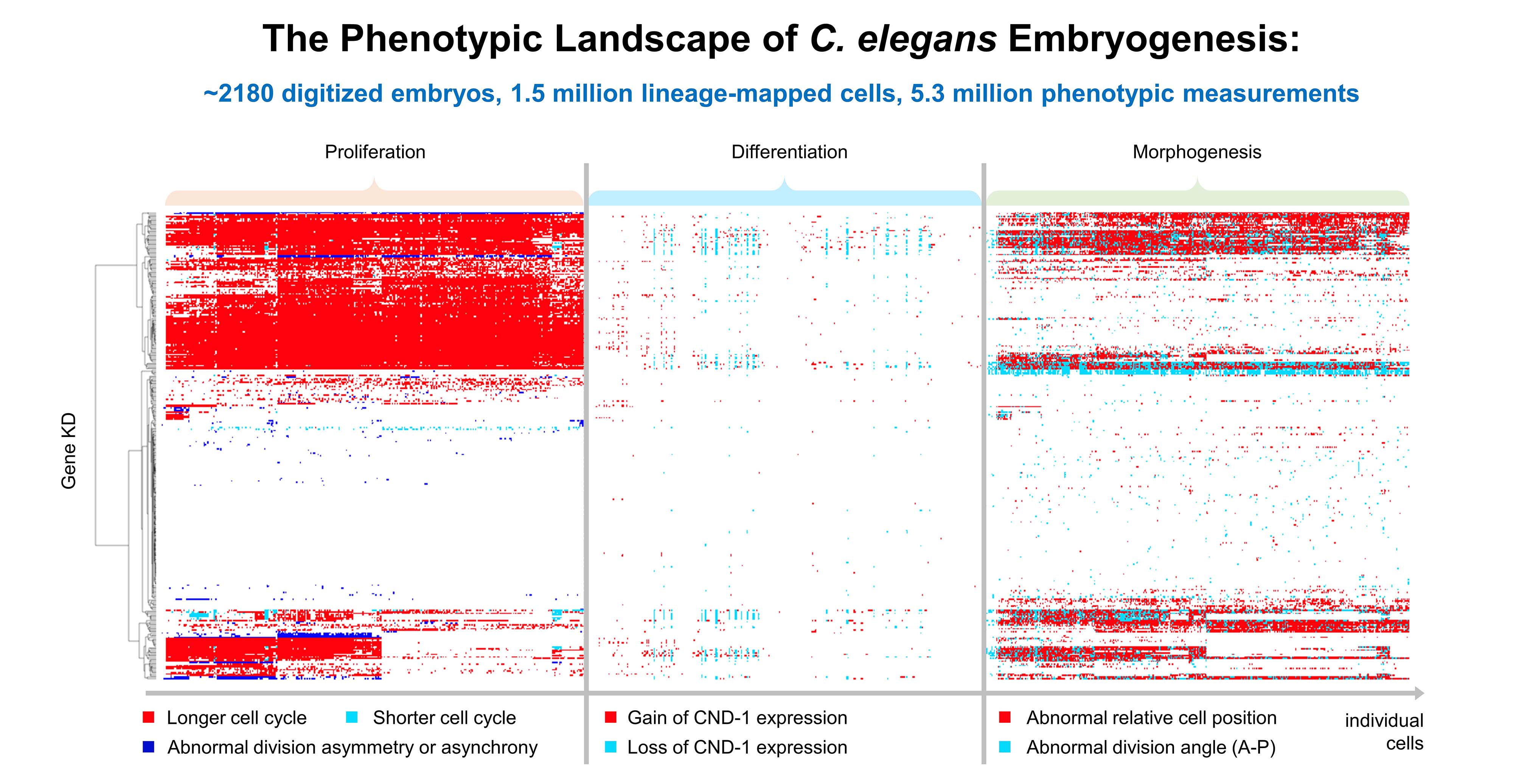
Developmental processes are intrinsically robust so as to preserve a normal-like state in response to genetic and environmental fluctuations. However, the robustness and potential phenotypic plasticity of individual developing cells under genetic perturbations remain to be systematically evaluated. Using large-scale gene perturbation, live imaging, lineage tracing, and single-cell phenomics, we quantified the phenotypic landscape of C. elegans embryogenesis in >2,000 embryos following individual knockdown of over 750 conserved genes. We observed that cellular genetic systems are not sufficiently robust to single-gene perturbations across all cells; rather, gene knockdowns frequently induced cellular defects. Dynamic phenotypic analyses revealed many cellular defects to be transient, with cells exhibiting phenotypic plasticity that serves to alleviate, correct, and accommodate the defects. Moreover, potential developmentally related cell modules may buffer the phenotypic effects of individual cell position changes. Our findings reveal non-negligible contributions of cellular plasticity and multicellularity as compensatory strategies to increase developmental robustness.
Mapped the single-cell phenotypic landscape of C. elegans embryogenesis
The majority of conserved gene knockdowns induce cellular defects
Cellular plasticity buffers against defects and increases developmental robustness
Multicellularity accommodates the phenotypic outcomes of cell position defects